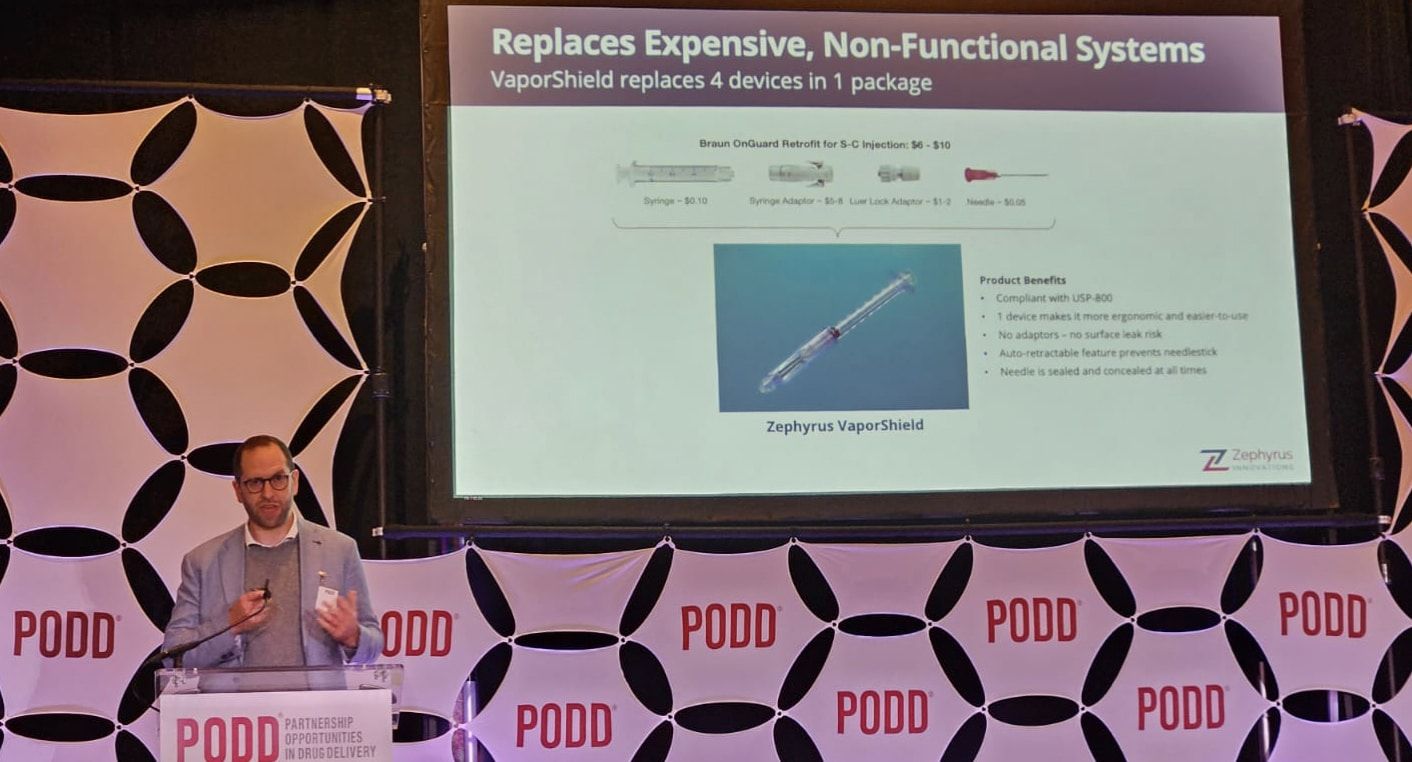
Zephyrus Innovations Announces First in World Injectable Closed System Transfer Device
Vancouver, Canada – 16 December 2024: Zephyrus Innovations (Zephyrus), a privately-owned medical device company designing and manufacturing safety syringes and Closed System Transfer Devices (CSTDs), announced its latest product, VaporShield, the world’s first injectable closed system transfer device (CSTD), at the Partnership Opportunities in Drug Delivery (PODD) conference in Boston, MA last month.
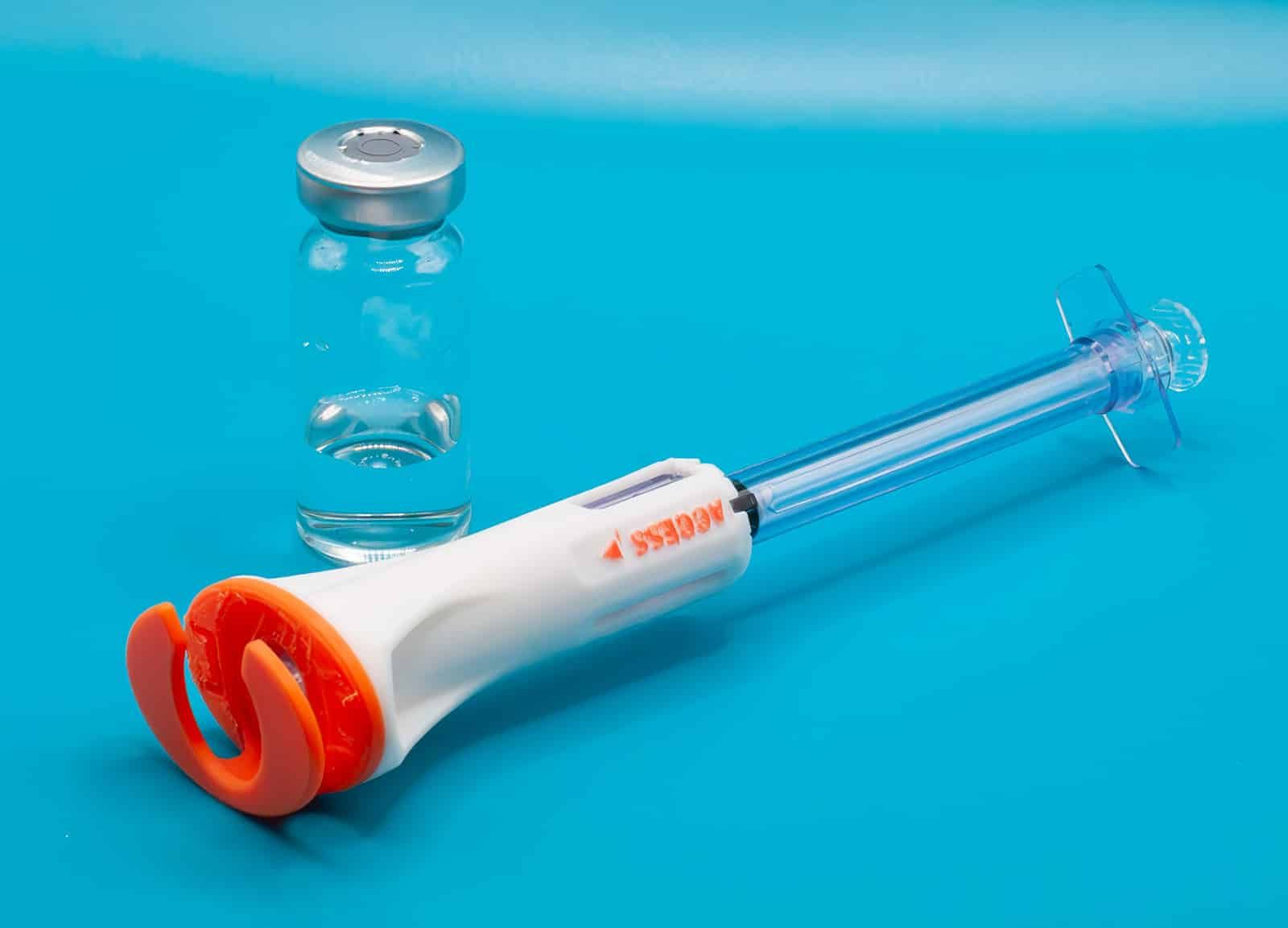
CSTDs are critical to protecting healthcare workers from hazardous drug exposure, which is now legally required in the US under USP800 legislation. Currently, there are no options that meet this legislation for healthcare workers who deliver subcutaneous or intra-muscular injections. The VaporShield provides a true closed system all the way from drug draw, to injection and through to disposal, while retaining the crucial safety syringe features of our Aeroject auto retractable syringe to stop needlestick injuries.
Guy Reynolds, Chief Executive Officer of Zephyrus, said: “VaporShield is a truly game-changing device. The seriously negative health impacts caused by exposure to hazardous drugs are well documented but unfortunately, there has been no device that fully protects healthcare workers giving these drugs via subcutaneous or intra-muscular administration, until now.”
“As we look towards FDA Clearance in the coming months, we can see significant opportunity to protect millions of healthcare workers around the world as they perform their day-to-day jobs.”
Zephyrus’ syringes are for general medical use in healthcare facilities by medical professionals for paediatric and adult population patients for aspiration and injection of fluids.